Identify Diagnostics Drug Test Result Forms

DRUG TEST RESULTS FORMS
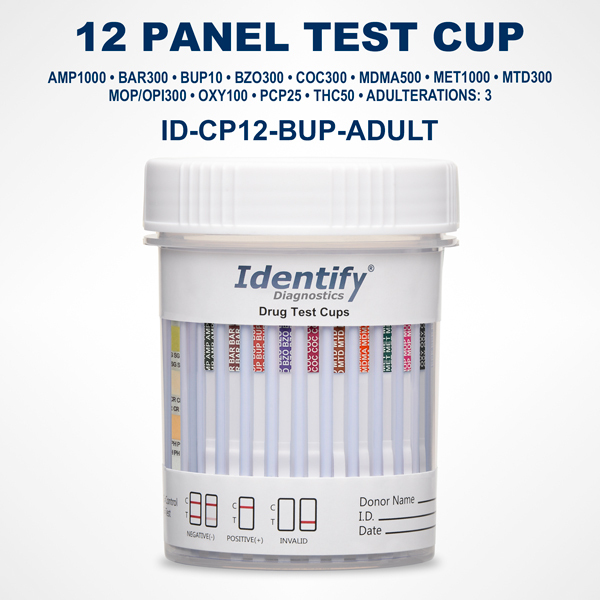

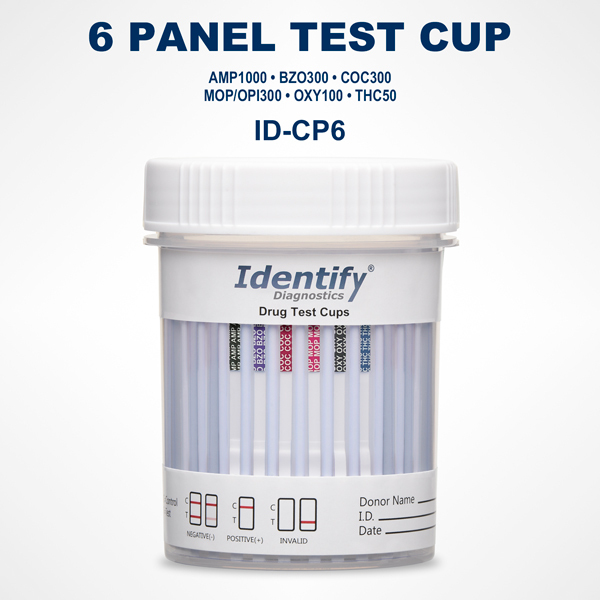
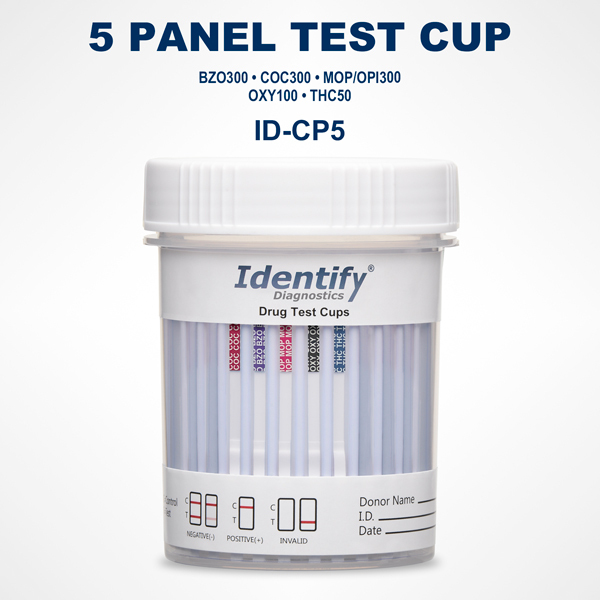



The Necessity and Usefulness of Identify Diagnostics Drug Test Result Forms
Identify Diagnostics drug test result forms are essential tools for ensuring accurate, consistent, and professional documentation when using Identify Diagnostics urine drug test cups, dip cards, or cassette devices. These forms are specifically tailored to match the configuration of each test device, helping users record and interpret results with greater confidence.
Device-Specific Accuracy
Each result form is aligned with a specific Identify Diagnostics drug test device. Whether the test cup screens for 5, 10, 12, or more drugs, the layout of the form mirrors the panel arrangement on the cup itself. This reduces the chance of mislabeling or misreading a result and supports clear, organized interpretation.
Improved Record-Keeping
The forms include dedicated fields for critical information such as donor ID, date, time, test administrator name, and individual test results. This supports thorough and professional record-keeping for workplaces, clinical settings, treatment facilities, or legal environments that require formal documentation of testing procedures.
Supports Regulatory Compliance
Using Identify Diagnostics result forms helps facilities comply with CLIA standards and other applicable regulations. The forms provide a reliable method of logging test outcomes, which can be essential for internal audits, external inspections, or program accountability.
Accessible and Convenient
These printable result forms are available as PDFs directly from the product page. Users simply click on the Identify Diagnostics brand icon to view a list of supported test devices. From there, selecting the correct device image opens a downloadable form tailored specifically for that product.
Enhanced Testing Confidence
By providing a standardized format for documenting results, these forms reduce interpretation errors and promote greater consistency across staff or multiple testing locations. They also provide a paper trail for follow-up decisions and improve communication among professionals involved in care, compliance, or employment decisions.
