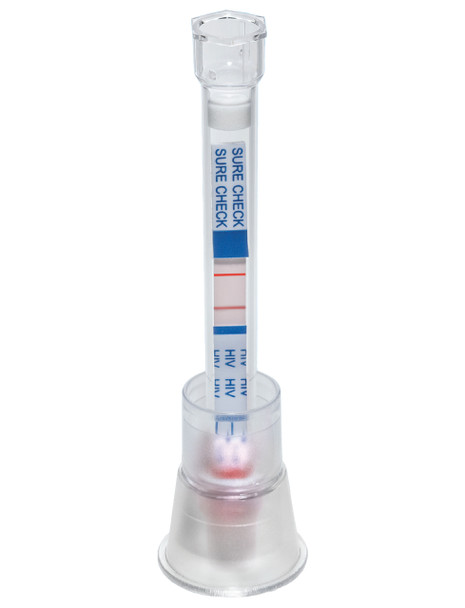
Sure Check
SURE CHECK® HIV 1/2 Assay - FDA Approved, CLIA Waived - 25 Tests
- SKU:
- 60-9507-0
- Availability:
- Ships in 3-4 business days. SIGNATURE required delivery.
- Shipping:
- Calculated at Checkout
Description
SURE CHECK® HIV 1/2 Assay (25 Tests) FDA Approved, CLIA Waived
Availability:
Ships in 3-4 Business Days
- FDA 510K Cleared / CLIA Waived HIV Test
- Purchase in bulk or small quantities to meet your specific volume needs
- Join our flexible auto-ship program to help make reordering easy
- The expiration date is typically 11 months or longer of available shelf life
- For large orders please email a.lugo@medicaldistributiongroup.com or call (727)744-2967
For the Sure Check HIV test package CLIA Waived insert please click the icon below:![]()
The SURE CHECK® HIV 1/2 Assay is a reliable and efficient rapid test designed for the detection of antibodies to HIV-1 and HIV-2 in human blood. This test kit, containing 25 individual tests, is FDA approved and CLIA waived, ensuring its credibility and ease of use in various settings, including clinical laboratories, point-of-care sites, and even for home use under professional guidance.
Key Features:
FDA Approved: The SURE CHECK® HIV 1/2 Assay has been rigorously tested and approved by the FDA, ensuring it meets high standards for accuracy and reliability.
CLIA Waived: As a CLIA waived test, it is simple to use and does not require highly specialized equipment or extensive laboratory training, making it accessible for a wide range of healthcare providers.
Rapid Results: This assay provides quick results, typically within 15 minutes, allowing for timely diagnosis and prompt decision-making.
High Accuracy: The SURE CHECK® HIV 1/2 Assay is known for its high sensitivity and specificity, reducing the likelihood of false positives and false negatives.
Ease of Use: The test is designed for simplicity, with straightforward instructions that make it easy to administer and interpret the results.
Convenient Packaging: Each kit contains 25 tests, making it suitable for use in clinics and other healthcare settings where multiple tests may be needed.
How It Works:
The SURE CHECK® HIV 1/2 Assay operates on a simple principle of immunoassay. It detects the presence of antibodies to HIV-1 and HIV-2 in human blood. When a sample is introduced to the test device, it reacts with specific reagents that can indicate the presence of these antibodies, providing a clear result within minutes.
Usage Instructions
Sample Collection: Obtain a blood sample using the provided lancet
Application: Apply the sample to the test device as per the instructions
Wait: Allow the test to develop for the specified time, typically around 15 minutes
Read Results: Interpret the results according to the guidelines provided in the kit. A positive result indicates the presence of HIV antibodies, while a negative result suggests no detectable antibodies.
The SURE CHECK® HIV 1/2 Assay is an essential tool for quick and reliable HIV testing. Its FDA approval and CLIA waived status ensure its reliability and ease of use, making it an excellent choice for healthcare professionals and facilities looking to conduct efficient and accurate HIV testing.